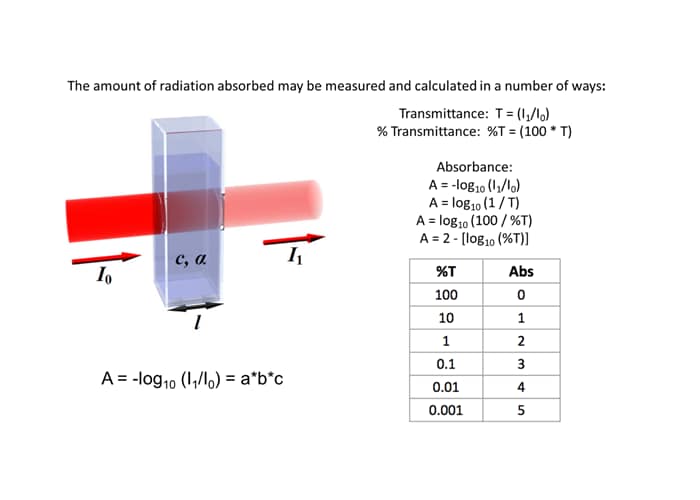
Spectrophotometry Absorbance Value . Transmittance, or light that passes through a sample, is usually given in terms of a fraction of 1 or as a percentage and is defined. A = log 10 (i 0 /i) where i 0 is the intensity of the incident light, and i is.
from www.ssi.shimadzu.com
The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector.light passing through a sample solution will partially be absorbed by molecules present in the sample.a spectrophotometer measures the amount of light that a sample absorbs.
How does a spectrophotometer measure a sample? Shimadzu Scientific
Spectrophotometry Absorbance Value A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength ( λ) by a sample, and can be. Deviation from linearity of beer’s law for two wavelengths where the molar absorptivities are (a) both 1,000, (b) 500 and 1,500, and (c) 250 and.a spectrophotometer measures the amount of light that a sample absorbs. Transmittance, or light that passes through a sample, is usually given in terms of a fraction of 1 or as a percentage and is defined.
From www.youtube.com
Absorption coefficient α calculation from UVVis absorbance data in Spectrophotometry Absorbance Value A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength ( λ) by a sample, and can be. The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector.a spectrophotometer measures the amount of light that a sample absorbs. A = log. Spectrophotometry Absorbance Value.
From www.analyzetest.com
A to Z of UVVis spectroscopy interpretation Spectrophotometry Absorbance Value A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength ( λ) by a sample, and can be.a spectrophotometer measures the amount of light that a sample absorbs. With the amount of absorbance known from the above equation, you can. Deviation from linearity of beer’s law for two wavelengths where the molar. Spectrophotometry Absorbance Value.
From www.bionity.com
Infrared (IR) Spectroscopy Spectrophotometry Absorbance Value The spectrum is characterized by the wavelength ( λmax) at which the absorbance (or molar. Transmittance, or light that passes through a sample, is usually given in terms of a fraction of 1 or as a percentage and is defined. The amount of light unable to.a spectrophotometer measures the amount of light that a sample absorbs. A =. Spectrophotometry Absorbance Value.
From fr.thptnganamst.edu.vn
Ntroduire 68+ imagen formule absorbance log fr.thptnganamst.edu.vn Spectrophotometry Absorbance Value A = log 10 (i 0 /i) where i 0 is the intensity of the incident light, and i is. The spectrum is characterized by the wavelength ( λmax) at which the absorbance (or molar.absorbance and % transmittance are often used in spectrophotometry and can be expressed by the following: The amount of light unable to.light. Spectrophotometry Absorbance Value.
From ar.inspiredpencil.com
Major Functional Groups Ir Absorption Spectrophotometry Absorbance Value Transmittance, or light that passes through a sample, is usually given in terms of a fraction of 1 or as a percentage and is defined.light passing through a sample solution will partially be absorbed by molecules present in the sample. The amount of light unable to.a spectrophotometer measures the amount of light that a sample absorbs.. Spectrophotometry Absorbance Value.
From organicchemistoncall.com
Most Commonly Used IR Spectroscopy Values In Organic Chemistry The Spectrophotometry Absorbance Value Deviation from linearity of beer’s law for two wavelengths where the molar absorptivities are (a) both 1,000, (b) 500 and 1,500, and (c) 250 and.light passing through a sample solution will partially be absorbed by molecules present in the sample. With the amount of absorbance known from the above equation, you can. The instrument operates by passing a. Spectrophotometry Absorbance Value.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Spectrophotometry Absorbance Value The spectrum is characterized by the wavelength ( λmax) at which the absorbance (or molar.the spectrum itself is a plot of absorbance vs wavelength ( a vs λ) or molar absorptivity vs wavelength ( ε vs λ ). With the amount of absorbance known from the above equation, you can. The amount of light unable to. A =. Spectrophotometry Absorbance Value.
From www.linquip.com
Beginners Guide What Is a Spectrophotometer Industrial Manufacturing Spectrophotometry Absorbance Valueabsorbance and % transmittance are often used in spectrophotometry and can be expressed by the following:light passing through a sample solution will partially be absorbed by molecules present in the sample. Deviation from linearity of beer’s law for two wavelengths where the molar absorptivities are (a) both 1,000, (b) 500 and 1,500, and (c) 250 and. A. Spectrophotometry Absorbance Value.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Spectrophotometry Absorbance Valueabsorbance and % transmittance are often used in spectrophotometry and can be expressed by the following: Transmittance, or light that passes through a sample, is usually given in terms of a fraction of 1 or as a percentage and is defined.a spectrophotometer measures the amount of light that a sample absorbs.light passing through a sample. Spectrophotometry Absorbance Value.
From www.vernier.com
Decoding Your Absorbance Readings Vernier Spectrophotometry Absorbance Value The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector. With the amount of absorbance known from the above equation, you can. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength ( λ) by a sample, and can be. The spectrum is. Spectrophotometry Absorbance Value.
From derangedphysiology.com
Absorption spectroscopy of haemoglobin species Deranged Physiology Spectrophotometry Absorbance Value With the amount of absorbance known from the above equation, you can. The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector. Deviation from linearity of beer’s law for two wavelengths where the molar absorptivities are (a) both 1,000, (b) 500 and 1,500, and (c) 250 and. A =. Spectrophotometry Absorbance Value.
From derangedphysiology.com
Absorption spectroscopy of haemoglobin species Deranged Physiology Spectrophotometry Absorbance Value A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength ( λ) by a sample, and can be. Transmittance, or light that passes through a sample, is usually given in terms of a fraction of 1 or as a percentage and is defined.a spectrophotometer measures the amount of light that a sample. Spectrophotometry Absorbance Value.
From www.researchgate.net
UVVis absorbance spectrum of melanin for 0.1, 0.2, 0.4, 0.6, and Spectrophotometry Absorbance Value Transmittance, or light that passes through a sample, is usually given in terms of a fraction of 1 or as a percentage and is defined. Deviation from linearity of beer’s law for two wavelengths where the molar absorptivities are (a) both 1,000, (b) 500 and 1,500, and (c) 250 and.light passing through a sample solution will partially be. Spectrophotometry Absorbance Value.
From studylib.net
Absorbance Spectrophotometry Analysis of FD&C Red Food Dye 40 Spectrophotometry Absorbance Value A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength ( λ) by a sample, and can be. The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector. Deviation from linearity of beer’s law for two wavelengths where the molar absorptivities are (a). Spectrophotometry Absorbance Value.
From inds.co.uk
SpectroVis Plus Spectrophotometer Spectrophotometry Absorbance Value The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector.light passing through a sample solution will partially be absorbed by molecules present in the sample. The amount of light unable to.a spectrophotometer measures the amount of light that a sample absorbs.absorbance and %. Spectrophotometry Absorbance Value.
From www.researchgate.net
Absorbance spectra of phenol red at different pH values. Download Spectrophotometry Absorbance Value A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength ( λ) by a sample, and can be.light passing through a sample solution will partially be absorbed by molecules present in the sample. A = log 10 (i 0 /i) where i 0 is the intensity of the incident light, and i. Spectrophotometry Absorbance Value.
From www.chegg.com
Solved 1. Consider the following two absorption spectra. Spectrophotometry Absorbance Value Deviation from linearity of beer’s law for two wavelengths where the molar absorptivities are (a) both 1,000, (b) 500 and 1,500, and (c) 250 and. The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector. A = log 10 (i 0 /i) where i 0 is the intensity of. Spectrophotometry Absorbance Value.
From www.odinity.com
Bromothymol Blue Spectrophotometry Report & Experiment Spectrophotometry Absorbance Value The spectrum is characterized by the wavelength ( λmax) at which the absorbance (or molar. The instrument operates by passing a beam of light through a sample and measuring the intensity of light reaching a detector.absorbance and % transmittance are often used in spectrophotometry and can be expressed by the following: With the amount of absorbance known from. Spectrophotometry Absorbance Value.